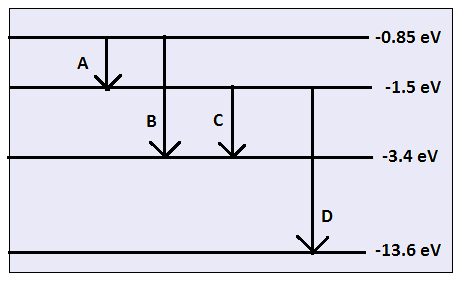
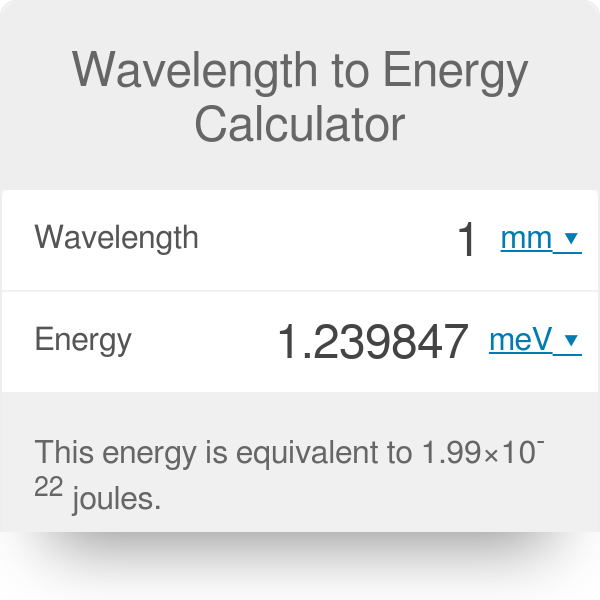
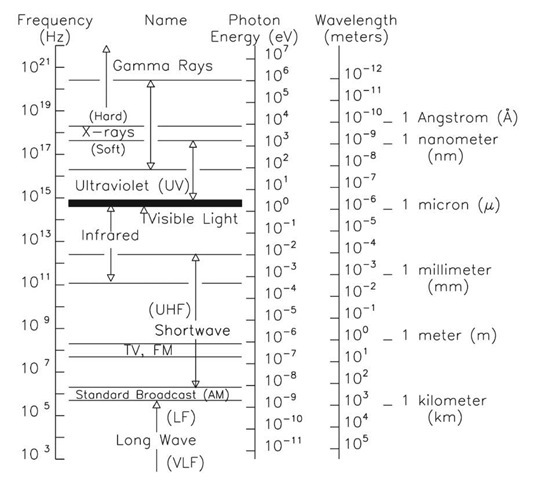
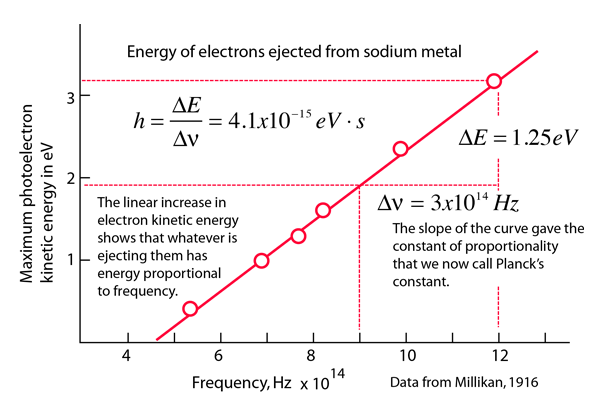
Calculating particle properties of a wave Ch. 12 A light wave consists of particles (photons): The energy E of the particle is calculated from the frequency. - ppt download
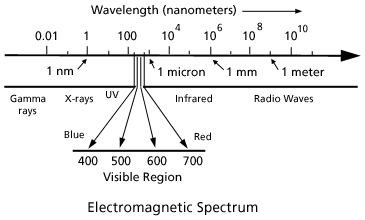
Optical Society of America: Exploring the Science of Light - Teachers and Parents: Articles: Color and Light

Derivation of hc=1240eVnm from SI units to eVnm + compute the energy of the Lyman-alpha photon in eV - YouTube

Question Video: Identifying an Electron Energy Level Transition Given the Wavelength of an Absorbed Photon | Nagwa

The maximum wavelength of light photoelectric effect from a metal is 200 nm. The maximum kinetic energy of electron which is emitted by the radiation of wave length 100 nm will be:
The work function of a metal is 10.0 eV. The longest wavelength of light that can cause photoelectron emission from this metal is approximately Options: a 248 nm b 124 nm b