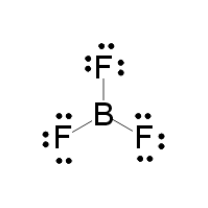
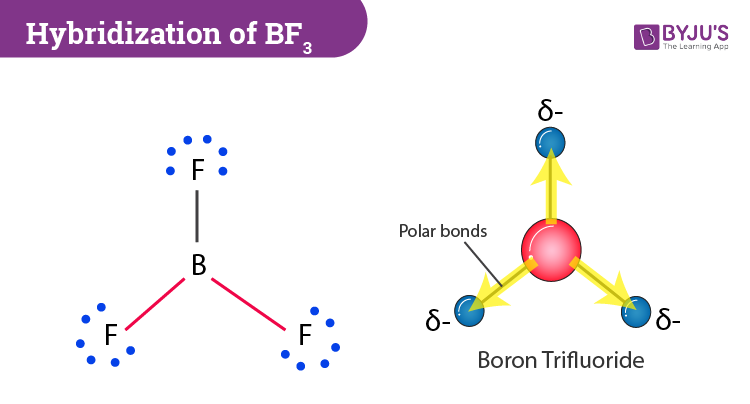
BF_{3} and NF_{3} both are covalent compounds but NF_{3} is polar whereas BF_{3} is non-polar. This is because :
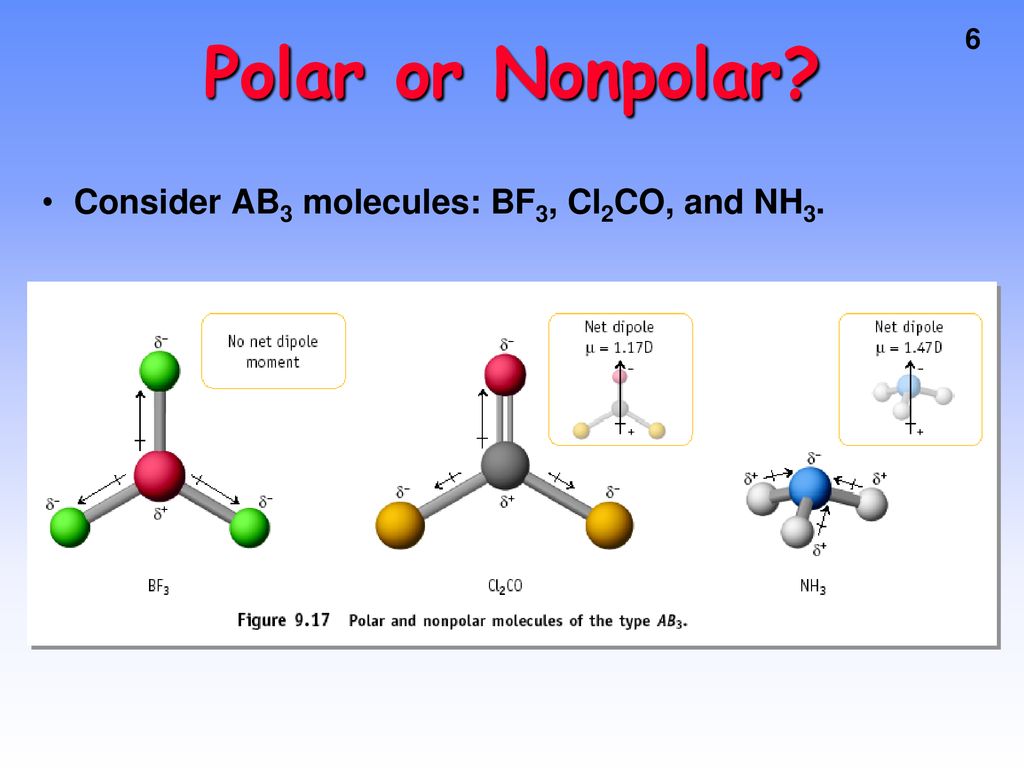
Ammonia (NH3) is a polar molecule while boron trifluoride (BF3), is a nonpolar molecule. What is the difference in the polarity of these compounds? - Quora

All three of the boron-fluorine single bonds in bf3 are polar. in which direction should the polarity - brainly.com

Boron Trifluoride BF3 is a non polar molecule whereas ammonia NH3is a polar molecule. The difference in polarity is related to the fact that

Which of the following is a false statement about BF3? A. BF3 has trigonal planar molecular geometry. B. BF3 has trigonal pyramidal electronic geometry. C. All three bond angles in BF3 are